Cavett test is used for the estimation of ethanol levels in biological samples like blood, urine, and saliva. The test is based on the oxidation of potassium dichromate for the estimation of alcohol.
However, the test uses nearly the same reagent as the Kozelka and Hine, but the procedure of performing the test is different.
What is the Cavett Test?
Cavett test is based on nearly the same principle on which the Kozelka-Hine test depends. In fact, the Cavett test for alcohol estimation was first developed in 1938 by J W Cavett.
Though they have the same reagent, there in their procedure and also in the sample quantity.
In the Cavett test, a very little amount of sample is needed about 1/10th of the sample that the Kozelka-hine test required. That’s the reason why it is also called to be the micro method of blood estimation.
As stated earlier, the Cavett test can be used for the determination of ethanol in biological samples such as blood, urine, and saliva.
Principle of Cavett Method
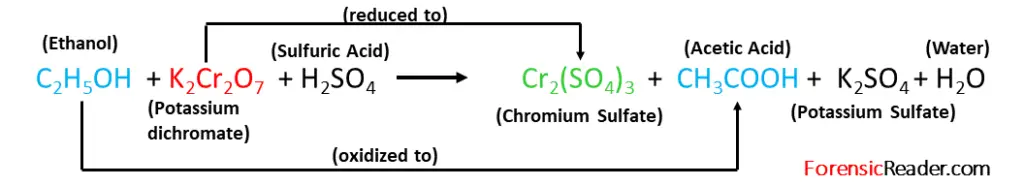
The principle of this method is based on the redox reaction between the potassium dichromate ions and ethanol.
In this, the dichromate goes through reduction and gives chromium sulfate. While alcohol gets oxidized to ethanoic acid.
So, the extent of conversion of ethanol in the presence of oxidizing agent to ethanoic acid is directly related to the alcohol content in the biological sample.
The Cavett test is governed by the following reaction
Procedure for Cavett Test
Before jumping to the procedure of this test, let’s have a close look at the following reagent.
Reagent Required
- Biological sample: Blood (0.1ml), Urine (0.2ml), and Saliva (0.1ml)
- Concentrated sulfuric acid
- Methyl orange: 0.1% in 0.025 N sodium hydroxide
- Ferrous sulfate 20% in 120 mL of concentrated sulfuric acid per liter.
- Reducing solution (titration): Red color of 15 mL methyl orange+ sulfuric acid + 1 mL of ferrous sulfate.
Step 1: Sample Preparation:
Place the blood sample in specialized glass stoppered flasks that already have standard potassium dichromate and sulfuric acid solution.
Step 2: Heat the Cavett’s Glass Flask
Now, the whole Cavett apparatus is heated in a water bath for 20-30 minutes.
Step 3: Titration Part
After heating, in order to determine the excess potassium dichromate, titration is performed.
Before titrating the sample, cool the sample to room temperature.
Now, titrate the excess dichromate against the prepared red color solution (15 mL methyl orange + sulfuric acid + 1 mL of ferrous sulfate).
Titrate the sample to the first pink tinge as the endpoint.
Limitation of Using Cavett Test
Following are the limitation of Cavett’s method of alcohol level estimation:
- False +ve results in decomposed bodies or live person if he/she is suffering from diabetes or ketoacidosis.
- It is inconsistent in reproducibility and harder to analyze multiple samples.
- Reagents are corrosive and need to be handled with care.
Reference
- J.W. Cavett, The determination of alcohol in the blood and other body fluids, 1938. [Translational Research Journal]
- Essentials of Forensic Medicine and Toxicology by Anil Aggrawal [Book]
- The Essentials of Forensic Medicine and Toxicology by Narayan Reddy [Book]
Also Read:
- Orthotolidine Test (Kohn & O’Kelly test): Principle, Reagent And Procedure
- Teichmann Test (Hematin) For Blood: Principle, Reagent, Procedure, Pros and Cons
- Famous Cases Related to Diatoms: How Diatoms Helps in Solving Crime?
- Burtonian Line: Cause, Color, Mechanism, Symptoms

FR Author Group at ForensicReader is a team of Forensic experts and scholars having B.Sc, M.Sc, or Doctorate( Ph.D.) degrees in Forensic Science. We published on topics on fingerprints, questioned documents, forensic medicine, toxicology, physical evidence, and related case studies. Know More.
