Orthotolidine or O-tolidine test is one of the presumptive tests for blood detection by imparting blue (or yellow, in high acidic medium). The reaction is totally based on the activity of hydrogen peroxides with the heme component of blood.
Moreover, the O-tolidine test was brought to be in practice as the replacement for the benzidine test.
That’s what the basics of an ortho-tolidine test. Let’s tackle it’ principle, color, reagent, and how to perform this test in real.
What is Orthotolidine Test?
Orthotolidine is a presumptive test for the detection of blood in biological samples such as urine, feces, and blood itself.
The test is based on the formation of the color blue as the sign of blood with the action of hydrogen peroxidase in a slightly acidic medium (pH 4.7).
However, if the acidity is less, say with 10% acetic acid, it produces a yellow color.
Other names: Kohn & O’Kelly test
Chemical Properties and Structure of O-tolidine
- IUPAC Nomenclature: 4,4′-diamino-3,3′-dimethyl-biphenyl
- Other Chemical Name: Orthotolidine, 2-Tolidine
- Chemical Formula: (C6H4(CH3)NH2)2
- Melting point: 126.5°C
- Color: Colorless
O-tolidine Vs O-toluidine Test
Both seem alike, but O-tolidine test is different from the O-toluidine Test.
- First, there is an additional ‘u’ in “O-toluidine” and
- Secondly, O-toluidine is used for the detection of chloride ions in water, not for blood. They are majorly used by pool cleaning facilities.
Also Read:
- Cavett Test of Ethanol Estimation And Forensic Importance
- Kozelka And Hine Method of Alcohol Determination
History and Necessity of Orthotolidine test
Ruttan and Hardisty, in 1912-13, introduced the orthotolidine test for the detection of blood (hemoglobin) in feces and urine. But it wasn’t widely accepted because of the benzidine test and misconception that it is too sensitive.
Later in the 1950s, when the benzidine fumes from the benzidine test were found to be carcinogenic, a need for new blood detection techniques. was raised.
And in 1952, J. Kohn, and T. O’Kelly, highlighted their reviews on the use of ortho-tolidine test over benzidine for bloodstain detection in one of the prestigious journals—British Medical Journals.
They stated that Analar ortho-tolidine test is more desirable than benzidine in terms of health hazard, with an acceptable level of sensitivity.
From the day after, this test is known to be Kohn and O-Kelly Test
But in 1974, o-tolidine was also found to be carcinogenic in rats. This is because o-tolidine based dyes are metabolized to their parent compound benzidine.
Eventually, in 1992, it was replaced by the Tetramethylbenzidine (TMB) test (our website).
Principle of O-Tolidine Test (Kohn Test)
The principle of this test is based on the oxidation reaction that can be catalyzed by heme to produce a blue coloration under slightly acidic conditions.
This chemical color test consisted of two different sets of reactions to give the final blue color (or yellow).
Stage 1: No Color Reaction
In the first stage, a drop of orthotolidine is added to the biological sample and no color changes are seen.
Stage 2: Action of Peroxide
As soon as the droplet of hydrogen peroxide is added to the sample diluted with o-tolidine, a bluish-green color is produced.
The color change is produced if the sample contains a heme compound, which in the majority of cases, is a sign of blood.
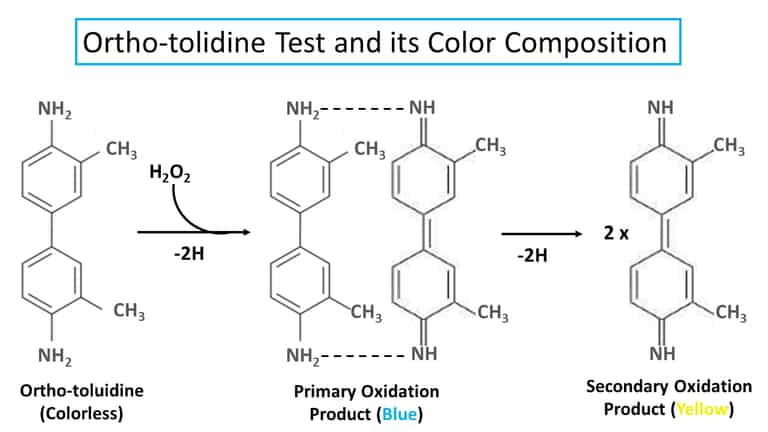
But in case the pH of the solution is brought les than pH 2, then the ortho-tolidine on reaction with heme produces a yellow color.
Reagents and Their Preparation
In the Ortho-tolidine test, there are a total of four reagents required. These are:
1. Preparation of Ortho-tolidine Reagent
Method 1: Take 0.25g of Analar Ortho-tolidine and dissolve in 80 ml of glacial acetic acid followed by the addition of 20 ml of water.
Mix well and keep the solution in a cool dark place. The shelf life of the solution is eight to 12 weeks. But as per guidelines, the examiner can use the solution for up to 2 weeks.
Method 2: Take 4% ortho-tolidine and add ethanol. Now, to make a working solution, add an equal amount of glacial acetic acid and water. followed by the addition of 20 ml of water.
Mix well and keep the solution at 4°C.
2. Hydrogen Peroxide Solution
Take 2 ml of 60 vol% solutions of hydrogen peroxide and make it to 100 ml with distilled water. The solution can be used for up to 6 days.
3. Sample Solution
O-tolidine test is basically used at the crime scene as a spot test, but it may be possible that it can be used in the laboratory.
So, for these cases, blood samples should be preserved by the addition of sodium citrate (anticoagulant).
If an examiner has to perform this test on preserved blood, then it should be first centrifuged for a better result.
4. Diluent
Citrate Buffer (pH 4.69) or Acetate buffer(pH 4.63) can be used as a diluent. If you’re preparing the solution of ortho-tolidine from method 1, then you can also use distilled water as a diluent. They can be used for obtaining the blue color in the orthotolidine test.
Note: If the examiner wants to obtain the yellow color (secondary oxidation product), the 10% acetic acid diluent is used.
Procedure for O-Tolidine Test
The following is a step-by-step guide on how you can perform an ortho-tolidine test for blood.
Procedure A: From liquid Blood
- Take 1 ml of o-tolidine solution in the test tube.
- To it, 0.02 ml of blood diluted sample is added.
- Agitate the test tube for a minute and leave it on the stand for 2 minutes.
- Now, add 1ml of hydrogen peroxide to the test tube.
- Swirl the test tube and leave it on the test tube stand for 10 minutes.
- Now, add 10 ml of water (you can also use 10% acetic acid to obtain yellow color).
Procedure B: From Dried Blood Stain
- Dip 2-3 swabs in O-tolidine solution for a minute.
- Now, scrape the surface of the dried blood stain with o-tolidine dipped swabs.
- Using a droplet, one drop of hydrogen peroxide is added to each of the swabs.
- After 5 minutes, drop all the swabs in the solution of the water.
Please Note: Test for fecal occult blood. If the sample is feces, take a pea-sized portion and add 5 ml of water. Boil the test tube with a swirl for 1-2 minutes.
Observations
In the case of water as the diluent, the blue color is obtained as the sign of blood.
While, if 10% acetic acid is used as a diluent, the yellow color is obtained as the screening color test for blood.
Note: Because of the corrosive nature of acetic acid (ph 1.5), it is not majorly employed. Hence, the O-tolidine test is generally stated using a blue color test for the bloodstain.
Spectrophotometric Properties of O-Tolidine Test Observation
Similar to benzidine, ortho-tolidine also has two oxidation products with different colors.
1. Primary Oxidation Product
- Blue color
- Maximum absorption peak at 630 mμ (millimicron).
- They are seen in high pH values of 4.6.
2. Secondary Oxidation Product
- Yellow color
- Maximum absorption peak at 435 mμ (millimicron).
- Seen in low pH values of 2.2 or less.
The following graph shows the o-tolidine test changes with the change in pH values.
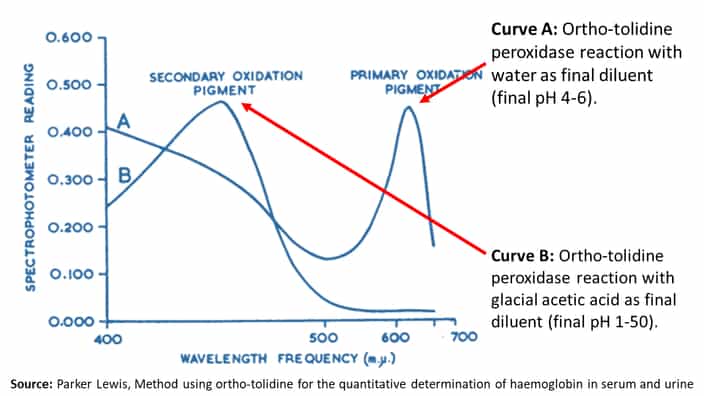
With the graphs, it is clear that the low pH values facilitate the secondary oxidation product while the high pH values produce the primary oxidation product.
Sensitivity of OrthoTolidine Test
- Immediate Result: Blood up to 1:10,000 gave an immediate positive blue color reaction.
- Within 20 seconds: Blood with dilution up to 1:100,000 gave positive but not immediate. They usually take up to 20 seconds to impart color.
Orthotolidine tests seem to be less sensitive than the tetramethylbenzidine (TMB) test (check sensitivity). As it can produce positive characteristic color within a dilution up to 1:10,000. And in some cases (bloodstain on cotton), can able to develop a color change in a blood dilution ratio of 1:500,000.
The following table states the sensitivity in terms of blood dilution and time taken to produce a characteristic blue color.
| Blood Dilution | Result | Time Taken |
| 1:50 | Positive | Immediate |
| 1:100 | Positive | Immediate |
| 1:500 | Positive | Immediate |
| 1:1000 | Positive | Immediate |
| 1:10,000 | Positive | Immediate |
| 1:50,000 | Positive | Within 5 secs |
| 1:100,000 | Positive | Within 20 secs |
| 1:500,000 | Positive (cotton cloth) | Within 20 secs |
| 1:1000,000 | Negative | NA |
False Positive Result in Orthotolidine Test (Specificity)
False Positive results of the o-tolidine test are seen in:
- Fruits: apricot, cantaloupe, banana, peach, pear, pineapple, Tangerine orange, watermelon.
- Vegetables: Asparagus, Avocado, green bean, broccoli, cabbage, Capsicum, carrot, cauliflower, celery, corn, cucumber, eggplant, garlic, lettuce, mushroom, sweet potato, radish, spinach, tomato, turnip root, and white onion.
- Rust and metals ions of copper, cobalt, iron, manganese, and nickel
- Milk (because of peroxidase enzyme)
- Body fluids such as sputum, pus.
Advantages of Orthotolidine Test
- Compatible sensitivity to benzidine test (1: 300,000).
- Easy to perform and economical.
- Less time-consuming.
- If screening is negative, no need to perform confirmatory tests.
- Less carcinogenic than benzidine test.
- One-time reagent preparation can be used for multiple tests for weeks.
Disadvantages of O-Tolidine Test
- Though high sensitivity but less specificity. So, a confirmatory test is needed to perform.
- A lot of false-positive results.
- In a 1972 study, it is also proven to be carcinogenic.
- Corrosive and acidic conditions needed to handle with care.
General FAQ
1. Orthotolidine test is used for determination of:
- Blood
- Saliva
- Semen
- All of the above
2. What are the reagents of O-tolidine test?
- 4% ortho-tolidine
- Hydrogen peroxide
- Glacial acetic acid
- Diluent
- All of the above
3. Who developed the method of detecting blood using the Orthotolidine test?
- Kohn
- O’Kelly
- Ruttan and Hardisty
- Both (1) and (2)
4. Which of the following sample can give a false-positive result in orthotolidine test?
- Horseradish
- Milk
- Rust
- All of the above
Reference:
- J. Parker Lewis, Method using ortho-tolidine for the quantitative determination of hemoglobin in serum and urine. 1965. [NIH.GOV]
- Kohn, J., and O’Kelly, T. 1952, British Medicine Journal.
- J. Kohn, and T. O’Kelly, An ortho-tolidine method for the detection of occult blood in feces, 1954[NIH.GOV]
- Forensic Biology by Richar Li [Book]
- Forensic Science Reform: Protecting the Innocent by Wendy J. Koen [Book]
- Essentials of Forensic Medicine and Toxicology by Anil Aggrawal [Book]
Further Reading:
- Tetramethylbenzidine (TMB) Test: Principle, Reagent and Procedure
- Wagenaar Test: Procedure, Reagents, Forensic Importance, Pros, and Cons
- Teichmann Test (Hematin) For Blood: Principle, Reagent, Procedure, Pros and Cons

FR Author Group at ForensicReader is a team of Forensic experts and scholars having B.Sc, M.Sc, or Doctorate( Ph.D.) degrees in Forensic Science. We published on topics on fingerprints, questioned documents, forensic medicine, toxicology, physical evidence, and related case studies. Know More.
