Takayama test is one of the micro-crystal confirmatory tests for the blood that is frequently used in forensic laboratories. It is also called Hemochromogen test, and a positive result is indicated by the pink feathery crystals of pyridine-hemochromogen.
Who Developed the Takayama Test?
It was first developed in 1912 by Masaeo Takayama, a Japanese forensic pathologist. Later, Takayama’s name has become the synonym for hemochromogen crystals.
Note 1: Takayama crystal is a type of Hemochromogen, specifically called to be pyridine-hemochromogen. The term ‘Hemochromogen’ was first coined by Stokes in 1864 when he prepared a characteristics spectrum product by reducing hematin with an alkaline solution. Later, it is also called to be “reduced hematin”.
What are Other Names of Takayama Test?
Other names are:
- Pyridine-Hemochromogen Crystal Assays Test
- Feathery Crystal Test
Also Read:
- Teichmann Test (Hematin): Principle, Reagent, Procedure, Advantages, and Disadvantages
- Presumptive Tests for Blood: 11 Important Forensic Test Included
Principle of Takayama Crystal Test
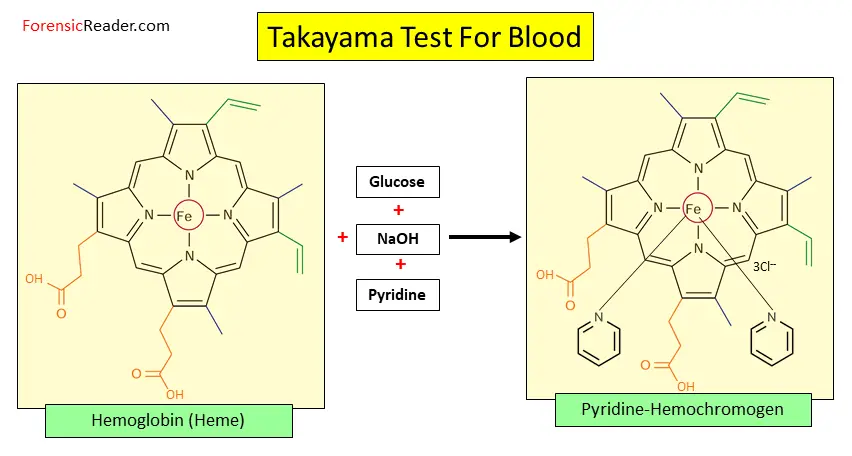
The principle is based on the formation of hemochromogen (or called pyridine ferroprotoporphyrin) with a reaction with pyridine.
In general, heme has six bonding sites of which four are attached to classic nitrogen bonding. While the other two sites also have N bonding but with the organic base of pyridine. In addition, the central iron atom has a 2+ charge i.e. Fe2+.
This altogether makes a hexa-coordination complex which has feathery pink structures that are known to be:
- Takayama crystals,
- Ferroprotoporphyrin,
- Pyridine hemochromogen crystals,
- Pyridine ferriprotoporphyrin crystals, or
- reduced alkaline haematin crystals.
The following are the reactions involved to form pyridine-hemochromogen crystals.
Reagents For Takayama Crystal Test
Following are the four reagents that are required for this test.
- Saturated glucose solution
- Sodium hydroxide (10%)
- Pyridine
- Water
Preparation of Takayama’s Reagent
This is how you prepare the reagent for the test:
- Take a test tube and pour 5 ml of saturated glucose solution.
- Add 5 ml of 10% Sodium Hydroxide solution.
- To this mix, add 5 ml of pyridine solution.
- Mix well.
- Now, add 10 ml of distilled water to the beaker and mix well.
Note 2: The common proposed ratio for the reagent preparation is: Saturated Glucose solution: Sodium Hydroxide: Pyridine: Water = 1:1:1:2. Although there are various compositions proposed for the Takayama test, this is one of the most popular and widely accepted ratios of reagents.
Preparation of Hemochromogen Takayama Crystals
Following is the step-by-step procedure for performing this test in the laboratory.
- Scrub the dried bloodstain sample and put it over a glass slide.
- Add 2-3 drops of prepared Takayama reagent.
- Cover the slide with a coverslip.
- Observe under a standard microscope.
Observations

A. Positive Takayama Result: In this test, there is a formation of:
- Feathery crystals
- Color: pink (red also)
- Arranged in clusters or sheaves.
It should appear within six minutes. If it doesn’t, warm the slide a little bit to fasten the reaction.
B. Negative Results: If no feathery crystals are formed within 30 minutes, the sample is not blood.
Advantages of Takayama Test
Advantages of the test are listed as:
- Best for old bloodstains.
- More reliable than Teichmann
- Small amount of stain sample is needed.
- Non-corrosive reagents
- No heating is required (majorly)
- It can develop hemochromogen crystals even if blood is exposed to freshwater decomposition media for 30 days [5].
Disadvantages of Takayama Test
Disadvantages of using this Pyridine-Hemochromogen test are:
- Take longer to perform.
- For full surety, the examiner has to wait for at least 30 minutes.
- No longer listed in State Crime Lab protocol.
Related MCQs
1. In Takayama test, ferrous iron from Hb reacts with pyridine to produce _______ crystals.
- Pink Rohmbic
- Pink Feathery
- Red Circular
- Green Feathery
2. Which of these tests is used to check for the presence of antibodies in blood-stained material?
- Lattes Crust test
- Ring Precipitin test
- Takayama test
- Teichmann test
3. Which of the following tests is a microchemical test for the detection of a bloodstain?
- Teichmann
- Leucomalachite green
- Phenolphthaleine
- Takayama
4. The preliminary examination of blood can be done by:
(i) Phenolphthalein test (ii) Precepitin test (iii) Takayama Crystal test (iv) Luminal test
Code:
- (i) and (ii) are correct
- (i) and (iv) are correct
- (ii) and (iii) are correct
- (i), (ii) and (iii) are correct
5. Which of the following is true for the Takayama reagent?
- Sodium Chloride, Glucose, and Sucrose
- Sodium Hydroxide, Pyridine, and Glucose
- Sodium Hydroxide, Glycerine, and Glucose
- Pyridine, Glucose and Sodium Chloride
References:
- Sourcebook in Forensic Serology, Immunology, and Biochemistry
- Anil Aggrawal, Essentials of Forensic Medicine and Toxicology
- Nordby, Forensic Science: An Introduction to Scientific and Investigative Techniques
- L.B.Miller, Hemochromogen Crystal Formation with Minute Amounts of Blood {ScienceDirect]
- Eriko, Takayama Test as a Blood Spot Test Tool in Blood Samples Exposed to Freshwater Decomposition Media [ResearchGate]
Continue Reading:
- Confirmatory Test For Blood: 7+ Important Forensic Medicine Tests
- Wagenaar Test: Procedure, Reagents, Forensic Importance, Pros, and Cons
- Tetramethylbenzidine (TMB) Test: Principle, Reagent and Procedure
- Cavett Test of Ethanol Estimation And Forensic Importance
- Danbury Tremor and Hatters Shake: Causes, Signs, and Symptoms

FR Author Group at ForensicReader is a team of Forensic experts and scholars having B.Sc, M.Sc, or Doctorate( Ph.D.) degrees in Forensic Science. We published on topics on fingerprints, questioned documents, forensic medicine, toxicology, physical evidence, and related case studies. Know More.
