Vacuum metal deposition (VMD) is probably one of the most sensitive fingermark detection and development techniques that are used in developing forensic fingerprints.
VMD is also capable of developing bloody fingerprints for nonporous surfaces as well as semi-porous surfaces like woods and some fabrics.
This article on “Vacuum metal depositions (VMD)” focuses on the history and development, components, and detailed explanations about its application in fingerprint development.
What is Vacuum Metal Deposition (VMD)?
Vacuum Metal Deposition i.e. VMD, is one of the most powerful latent fingerprint development techniques. It develops fingerprints by the process of metal deposition over the surfaces.
History and Development of Vacuum Metal Deposition (VMD)
1964: Tolansky while working on interference filters, stated that the vacuum system can develop the fingermarks using the silver metal deposition method.
1968: Theys et al. developed fingerprints on paper using a mixture of zinc, antimony, and copper powder, by vacuum metal deposition technique.
1972: Hambley concluded in his thesis that the best combination for developing fingerprints using vacuum metal deposition is gold or silver followed by another layer of cadmium or zinc.
1976: Kent et al. use industrial vacuum metal coaters for fingermark development on surfaces like polythene.
2001: An article in Forensic Science International, solves the challenges of the excess of deposition gold film on the surface of low-density Polyethene (LDPE) by vacuum metal deposition technique. (N Jones et al. 2001)
2001: N Jones et al. reported the factors that affect the normal and reverse development of latent fingerprints on the polyethylene substrate.
2007: Philipson and Bleay developed latent fingermarks on the plastic substrate by using a silver vacuum metal deposition method.
2007: Guraratne et al. reported that aluminum metal can be used for the development of fingerprints in combination with any secondary metal coating or deposition.
2010: J Fraser et al. show that vacuum metal deposition can be used for the development of fingermarks on the fabrics. They use nylon, polyester, polycotton, and cotton to develop prints using gold-zinc metals.
2011: Yu et al. show that the oxide of zinc (ZnO) can be used for developing latent fingerprints on polymer surfaces by the deposition of vapors of zinc oxide in the vacuum chamber.
2014: J Fraser et al. reported a comparative analysis of vacuum metal deposition and cyanoacrylate fuming for visualizing fingermarks on fabrics.
Also Read:
- Forensic Analysis of Diatoms in Drowning: Extraction and Procedure
- Henry Fingerprint Classification System: Key Major, Primary, Secondary and Subsecondary
- Battley Single Digit Classification System: Fingerprint Identification
- Draughtsman Appearance: Colonies Structure & Morphology
Theory and Principle of VMD
The theory of vacuum metal deposition is based on the condensation characteristics of metals over surfaces.
Metals like Cadmium, Zinc, and Magnesium can vaporize to form very fine particles which are further condensed to form a layer over the surface.
The theory of metal deposition is divided into two parts. These are
- A very thin invisible layer of Gold: Gold is first evaporated under a vacuum to form a very fine thin layer on the surface which is brought to be examined.
- Zinc layer making negative Fingermarks: The second layer of zinc is now evaporated to form a corresponding layer to the surface and zinc can’t able to diffuse into the fingermarks.
The ridges are therefore left transparent, while the background is plated with a layer of zinc in the case of normal vacuum deposition.
Under normal circumstances, VMD treatment produces negative marks, as zinc deposits on the background substrate and not the print ridges themselves.
Note: Cadmium can be used for the development of fingermarks, but due to its toxicity it is no longer used.
Types of Vacuum Metal Deposition
There are three types of Vacuum Metal Deposition methods, listed below:
- Normal Development
- Over Development
- Reverse Development
1. Normal Development
In normal development, the primary layer of gold covers the entire substrate. Gold gets diffused into the ridges of latent prints followed by zinc that covers the whole surface, except ridges.
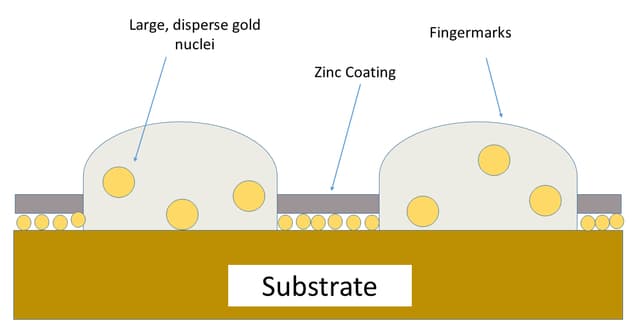
The developed prints have the entire surface covered with zinc except for the ridges. This creates a different contrast of friction ridges to the surroundings and hence prints are visible.
2. Over Development
This may occur in certain types of surfaces like HDPE (high-density polyethylene) when an excess amount of primary coating of gold gets deposited on the surface.
In overdevelopment, the gold clusters overgrow on the ridges and when the corresponding layer of zinc is introduced it covers the entire area even over the finger ridges.
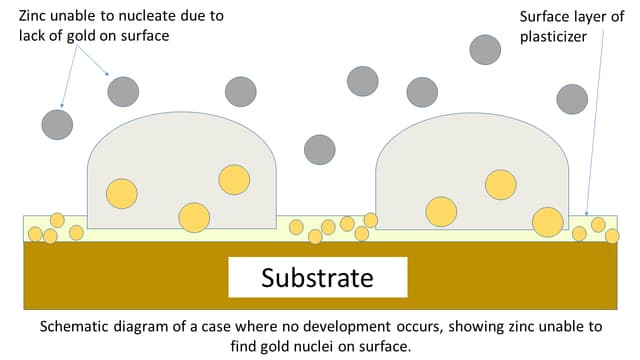
Then there is no clear ridge details are seen, and it becomes nearly impossible to develop clear fingerprints using this method.
3. Reverse Development
Surface-like polyethylene LDPE (low-density polyethylene) shows the reverse development of the fingermarks.
In this development, the secondary layer of zinc is deposited more rapidly on the latent finger ridges than the surface.
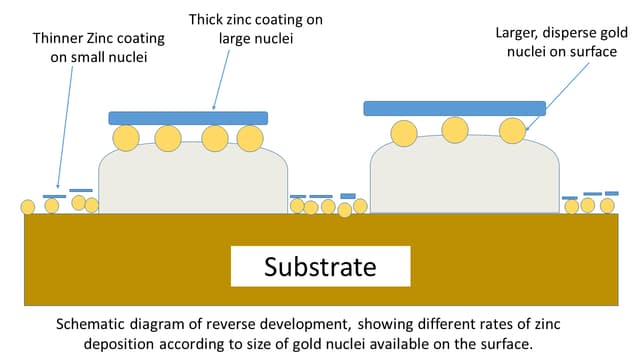
Theories to describe the reverse development
There are two theories to describe why the reverse development occurs in Vacuum Metal Deposition;
1. Based on the surface porosity
Porosity is the quality of being porous. If the surface has semi-porous or porous then, in this case, the gold nuclei diffused more through the porous surfaces.
And the resultant corresponding zinc development leads to reversing the development of fingerprints.
2. Prints are dried enough
In another explanation, it may be possible the fingerprints or contaminants like grease, are dried enough so that the gold particles are unable to diffuse into the fingermarks.
This results in the deposition of larger gold nuclei on the surface. Hence, after the application of the second layer, the zinc deposition is concentrated on the latent ridges and forms a thicker layer than the surface.
So, the resultant prints are reserved for normal development having a thicker zinc coating on the latent prints than the entire surface.
Process of using Vacuum Metal Deposition
The deposition process includes the formation of metal nuclei of different sizes that can be deposited on different regions of the surfaces.
In VMD, a very fine primarily mist vapor, mainly gold is used to create a very fine and thin layer on the surface.
Then the next layer is brought to be thicker which is developed by applying the corresponding layer of zinc metal.
Now discuss the development process in detail;
First stage: Layer of Gold
In this stage, gold metal or strips are used to be evaporated and resultant nuclei are very small but discrete in size that can’t be seen through the eyes. The gold nuclei form a very fine, thin, and discontinuous layer on the surface.
Example: One can co-relate the deposition of gold nuclei with the talcum powder on a table. Talcum powder forms a fine layer on the surface but is discrete and discontinuous to the surface.
In practicality, low pressure is maintained by facilitating less collision between the nuclei of gold. This is the key to even distributed coating on ridges.
When the gold nuclei are bombarded to the surfaces they serve two purposes. First, disperse over the entire surface, and secondly, start diffusing into the fatty acids.
As the gold film layer is very thin which is not visible, further development of the fingermarks is needed.
Properties of gold used in vacuum metal deposition
In VMD, 99% of gold metal is used in the form of cylindrical wires of 0.5mm diameter. And for a single deposition procedure, a 5-millimeter length wire is required. Moreover, the gold particle is typically heated at 1600° C at 10-4 Mbar.
2nd Stage: Layer of Zinc
The zinc layer allows the user to control the deposition factors because of higher chamber pressure and the ability of zinc to re-evaporate.
The more air pressure in the chamber serves the major purpose of maintaining the uniformity of the zinc layer across the surface.
Properties of Zinc used in VMD
Zinc has a melting point of around 420°C. When the pressure reduces the zinc starts to sublime i.e. evaporate directly to gas. Once the boiling point of zinc is reached the zinc particles agitate very fast.
As zinc starts to sublime, the zinc particles have a tendency to travel both via straight and curved trajectories depending upon the obstacles in the path.
So, even an obstacle like shutting the shutter does not stop the zinc particles from getting deposited on the surface.
Components of Vacuum Metal Deposition Unit
- A vacuum coating chamber
- Thickness Monitor
- Shutter
- Sample holder
- Baffle Valve
- Vacuum Direction Valves
- Rotatory Pump
- Diffusion Pump
1. Coating Chamber
The chamber is fitted with:
- one thickness meter and monitor,
- a sample holder,
- a shutter and
- a tungsten boat.
In this VMD chamber, the evaporation and deposition of metals occur.
2. Thickness Monitor
The thickness monitors are based on the principle of resonance frequencies and are built with a thin quartz film coated with gold from both sides.
When the deposition of metal on quartz film takes place, then there is a corresponding resonance frequency. And deposited mass is get converted into arbitrary units known as “counts”.
Moreover, the diameter is about 8mm to 10mm.
It serves two major purposes in a vacuum metal deposition chamber:
- It monitors the evaporation rate in terms of counts per second.
- Total number of counts
The thickness monitor is used to check only gold evaporation and deposition rate because zinc can re-evaporate after deposition.
3. Shutter
It is used to control the amount of gold deposition. The shutter operates with a programmed time period in accordance with the thickness monitor.
Initially, the shutter is kept closed until the gold gets evaporated. Then opened to bombarded gold nuclei to the surface. Typically shutter is programmed to open after the 30s of passing electric current to gold for heating.
4. Sample Holder
It is a removable, semi-cylinder that covers the half-vacuum metal deposition chamber. It can be aligned horizontally or vertically to the VMD chamber. The samples to develop are fixed with small magnets.
5. Baffle Valve
A large heavy valve is opened when the VMD chamber pressure is below the threshold pressure i.e. ~8 x 10-2 Mbar. It also prevents diffusion pump oil from oxidation.
6. Vacuum Direction Valves
There are two operational direction valves of the shutting and opening function to achieve the desired conditions required in vacuum metal deposition.
7. Rotatory Pump
It is a two-stage rotatory pump that is used to create vacuum pressure until the threshold pressure.
Note 2: A two-stage rotatory pump is a pump with two rotators and valves. The first stage creates the vacuums and the second stage cleans the system, which creates a deeper ultimate vacuum level.
8. Diffusion Pump
A diffusion pump contains oil chambers at the bottom which are used to pump oils to achieve and maintain the VMD chamber pressure.
When the oil is heated to its boiling point oil steam fills the cylinder and the fast oil molecules capture air molecules and concentrate them at the bottom of the pump.
Then, the coolant around the diffusion pump condenses the oil which then travels down to the oil sump.
Frequently Asked Questions on Vacuum Metal Deposition
What difference in Gold Layer and Zinc Layer film?
The gold vapors not only cover the whole surface of the sample but also penetrate the ridges marks of the fingerprint.
Whereas in the case of zinc particles, they are not able to diffuse into the friction ridges.
What are the factors affecting the development phases in the VMD chamber?
1. Presence of dirt, oil, grease, etc.
2. Surface is not even and has more than one chemical composition and oxidation state.
4. Scratches, abrasions, or cuts over the surface.
Why Gold is used as the primary layer in VMD?
This is because of the following reason;
1. The gold layer acts as the primer for the deposition of the secondary zinc layer.
2. As gold is not selective in nature because it gets dispersed on entire surfaces even on the ridges of fingermarks.
3. Because of gold’s Inert nature, it does not react with fingermarks and hence other evidentiary chemicals remain unaffected.
4. Without the initial layer of gold, zinc deposition may be inhibited by the presence of many greasy contaminants.
Why Zinc is used as the Secondary layer in VMD?
Reason 1: Zinc is used to trace down the finger ridge details by the unseen deposition of gold nuclei on the surface of ridges and surfaces.
Reason 2: The zinc particles get easily re-evaporated so that the deposition factors can be controlled. The controlled deposition factor is defined by the type of surface and the contract needed.
What happens to fatty acid in high vacuum vapor pressure?
The latent prints contain many fatty acids which are mostly insoluble in water and have a long-chain structure of fatty acids.
As the chain structure increases the vapor pressure of compounds decreases. This means that these fatty acids remain stable even in high chamber pressure.
Why shutter is not required in Zinc deposition?
The zinc particles have a tendency to travel both via straight and curved trajectories depending upon the obstacles in the path. So, even obstacle like shutting the shutter does not stop the zinc particles to get deposited on the surface.
Conclusion
Vacuum metal deposition is one of the best fingerprint development methods. However, the optimum development of fingerprints by Vacuum metal deposition method requires an appropriately configured VMD chamber (with a thickness monitor and shutter highly recommended), and an operator experienced in applying the technique on a range of different surfaces.
Read More:
- Magnetic Powders: Types, Principle, Fingerprint Development, Advantages, And Disadvantages
- Can you use a stamp pad for fingerprints? Regular Ink Pads Vs Fingerprint Ink Pads
- Can You Get Fingerprints From Leather Surface? [Forensic Expert Answer]
- How Long Do Fingerprints Stay on Clothes? A Forensic Guide
References:
- https://www.ncbi.nlm.nih.gov/pubmed/24630323 [NCBI]
- https://www.sciencedirect.com/science/article/pii/S0379073800003108 [URL]
- https://www.sciencedirect.com/science/article/pii/S0379073801005072 [URL]
- http://www.aimcal.org/uploads/4/6/6/9/46695933/aimcal-metallizing-technical-reference-5thed.pdf [URL]
https://www.sciencedirect.com/science/article/pii/0040609095068538 [ScienceDirect] - https://www.sciencedirect.com/science/article/pii/S0379073810003841 [ScienceDirect]

FR Author Group at ForensicReader is a team of Forensic experts and scholars having B.Sc, M.Sc, or Doctorate( Ph.D.) degrees in Forensic Science. We published on topics on fingerprints, questioned documents, forensic medicine, toxicology, physical evidence, and related case studies. Know More.
